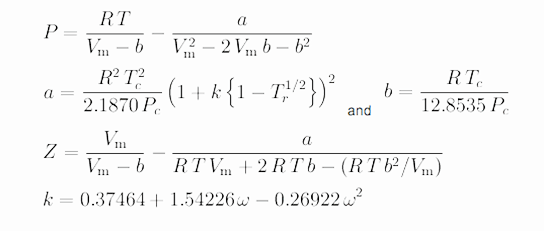
The familiar and most commonly known gas law is
PVm = RT
And the non ideal corrected is
PVm = ZRT
In the above P = Pressure , Vm is volume (molar volume of gas) Z = the compressibility factor , R = the universal constand and T is for temperature.
In conclusion of the above we can say that Z = PVm / RT which is the most commonly used EOS (Equation of State)
For those working in process industries such as natural gas production, petrochemical and refining the most commonly used (more developed) equations of state are
REDLICH- KWONG
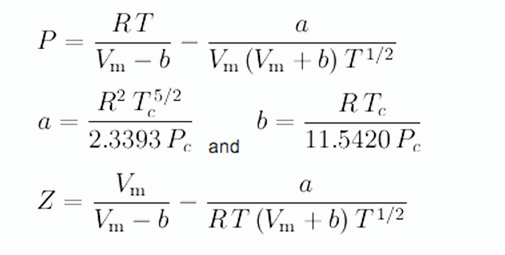
SOAVE-REDLICH-KWONG
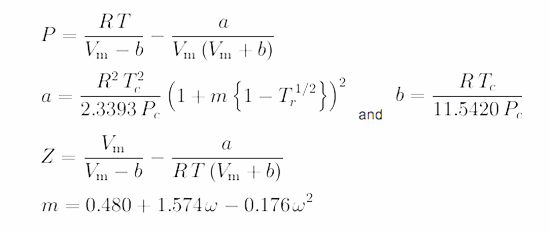
SOAVE-REDLICH-KWONG